Hydrochloric Acid and Sodium Hydroxide Balanced Equation With States
Aqueous sodium carbonate cobalt II nitrate Reaction Type. 2Na2H2O2NaOHH2 A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products.

Chapter 11 Matter And Change 11 1 Describing Chemical Reactions Ppt Download
Hydrochloric acid reacts with sodium hydroxide to form sodium chloride the salt and water.
. Balanced Molecular EquationHClaq NaOHaq -- NaClaq H2OlComplete Ionic EquationH aq Cl- aq Na aq OH- aq --Na aq Cl- aq H2O lCancel out the spectator ions. Aqueous solutions of barium hydroxide and hydrochloric acid and barium hydroxide and hypochlorous are. The balanced chemical equation is.
Split strong electrolytes into ions the complete ionic equation. For the reaction that occurs when aqueous solutions of hydrochloric acid and sodium hydroxide of hydroxide. The balanced chemical equation for HCL and NaCO3 is NaHCO3s Has Naas CO2g H2Ol.
Write the state s l g aq for each substance. The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation. Ammonium hydroxide reacts with sulphuric acid produces Ammonium sulphate and water.
The basic formulae hydrochloric acid isHcl and the basic formulae for sodium hydroxide is NaoH so the balanced reaction between these two components is. Write the balanced equation for this reaction. The reaction will occur as follows.
ΔH because that a neutralisation reaction is negative. Sodium chloride is also known as common salt. When acid and base react salt and water are produced.
Sulfuric acid alternative spelling sulphuric acid also known as vitriol is a mineral acid composed of the elements sulfur oxygen and hydrogen with molecular formula H 2SO 4. Once you know how many of each type. N a O H H C l N a C l H 2 O the equation is already balanced.
Sodium Hydroxide And Hydrochloric Acid Balanced Equation. Ii Barium chloride Aluminium sulphate Barium sulphate Aluminium chloride. The physical states of NaOH HCl and NaCl are solutions in water hence written as aq.
Write a balanced chemical equation with the state symbols for the following reactions. Product Names States if none why not. Equation is a chemical equation for this is H2SO4 plus Ba OH react.
The balanced chemical equation is. Write balanced chemical equation with state symbol for the following reaction sodium hydroxide solution react with hydrochloric acid solution to produce sodium chloride solution and water ds0847151 is waiting for your help. Include the state symbols.
H 2 Cl 2 2HCl. I H 2 Cl 2 HCl. Write the equation and balance it.
Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equations arrow. Write the balanced equation for the following chemical reactions. Chemistry questions and answers.
2 NH 4 OH H 2 SO 4 NH4 2 SO4 2 H 2 O. Sodium hydroxide solution in water reacts with hydrochloric acid solution in water to produce sodium chloride solution and water. The representation of a chemical reaction in the form of substances is known as a chemical equation.
Sulfuric acid and barium hydroxide and hydrochloric acid gives barium chloride and water. Write a balanced chemical equation with state symbols for thefollowing reactionsii Sodium hydroxide solution in water reacts with hydrochloric acid solution in water to produce sodium chloride solution and waterAnsweriiNaOH aq HCl aq NaCl aq H2O lThis an example of Neutralisa. The enthalpy change ΔH 2 to be -502 kJmol.
N a O H H C l N a C l H 2 O. Hydrochloric acid aqueous sodium hydroxide Reaction Type. When strong acids such as H C l hydrochloric acid reacts with strong base like N a O H sodium hydroxide then neutral salt such as N a C l sodium chloride and water are formed.
Product Names States if none why not. Sodiums hydrochloric acidaq - sodium chlorideaq hydrogeng Solution. The balanced equation tells us that 1 mole of Zn reacts with 2 moles of HCl.
This is the balanced equation of. The given reaction is as follows. H 2 O is water in liquid state written as l.
NaOH aq HCl aq NaCl aq H 2 O l We know that sodium hydroxide NaOH is a base and. Write the balanced chemical equation for the reaction in which aqueous sodium hydroxide is added to aqueous hydrochloric acid. Double-replacement combination combustion decomposition single-replacement.
In the reaction sodium chloride NaCl is formed. From above we can say that the reaction between hydrochloric acid and sodium hydroxide gives the sodium chloride and water. Be sure to include states of matter.
I We are given a reaction in which sodium hydroxide and hydrochloric acid react. Write the balanced chemical equation for following reactions a Dilute hydrochloric acid reacts with sodium hydroxide solution b Lead nitrate is heated Copper sulphate reacts with iron sulphate reaction d Heating a mixture of iron and sulphur e Passing chlorine through a solution of potassium bromide. NaOH HCl -- NaCl HOH So Sodium Hydroxide and Hydrochloric acid make Sodium Hydroxide and Water.
The limiting reactant is either the HCl or. To balance NaOH HCl NaCl H2O youll need to be sure to count all of atoms on each side of the chemical equation. Hydrochloric acid aqueous sodium hydroxide 7.
I Hydrogen Chlorine Hydrogen chloride. When an aqueous sodium sulphate solution reacts with an aqueous barium chloride solution. Chemical Reactions and Equations.
Iii Sodium Water Sodium hydroxide Hydrogen. Hydrochloric Acid And Sodium Hydroxide Balanced Equation. I BaCl2 Na2SO4 BaSO4 2NaCl.
The written and balanced equation is. N a O H a q H C l a q N a C l a q H 2 O l. Chemical equation The reaction is an example of a reaction.
Ag aq NO3 - aqH3O aqCl - aq AgCls H3O aqNO3 - aq A precipitate is formed and the str. This reaction states that 1 molecule the HCl will certainly react with 1 molecule of NaOH to produce 1 molecule of the salt sodium chloride NaCl and also 1 molecule the water.

Caco3 Naoh Hcl Nahco3 Cacl2 H2o Balanced Chemical Equation
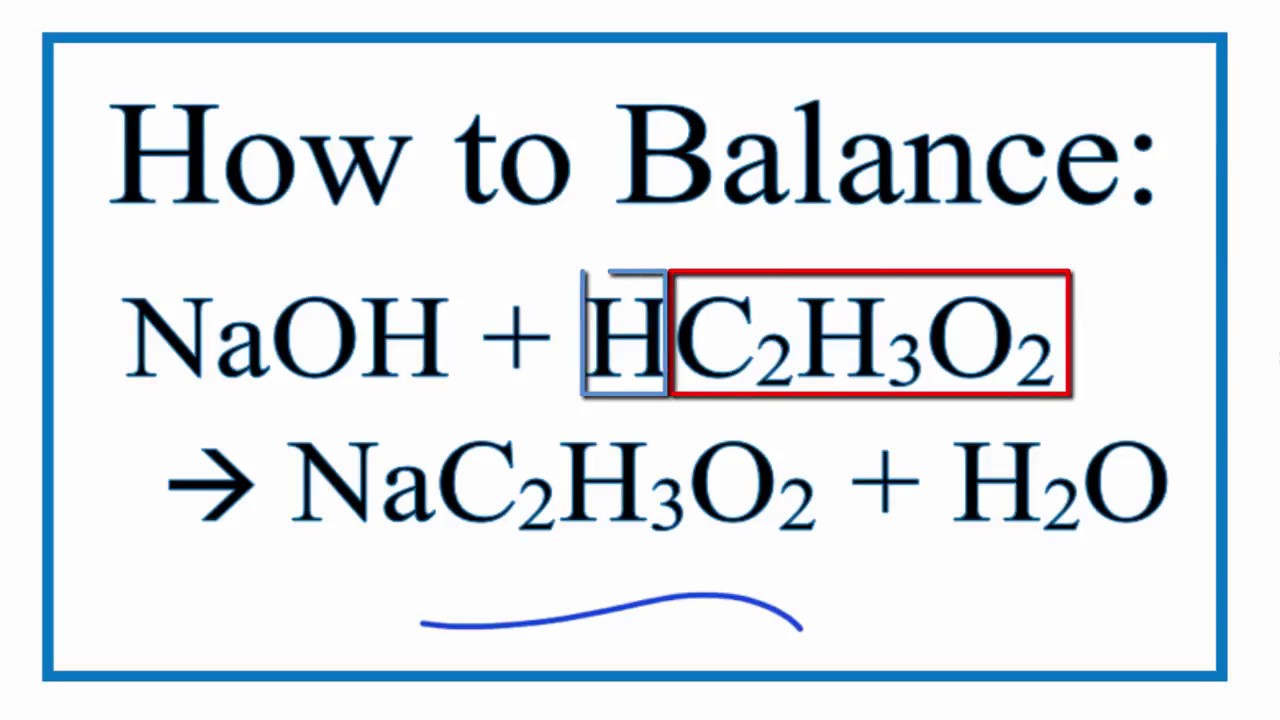
How To Balance Naoh Hc2h3o2 Nac2h3o2 H2o Sodium Hydroxide Plus Acetic Acid Youtube

How To Balance H3c6h5o7 Naoh Na3c6h5o7 H2o Youtube

Question 3 Write A Balanced Chemical Equation With State Symbols For The Following Reactions I Solutions Of Barium Chloride And Sodium Sulphate In Water React To Give Insoluble Barium Sulphate And The

Type Of Reaction For Naoh Hcl Nacl H2o Youtube
How To Complete And Balance An Equation For Reaction Of Hydrochloric Acid Hcl With Sodium Hydroxide Naoh Quora

Ionic Equations A Chemical Equation Shows The Number Of Atoms And Molecules Of The Reactants And Products Also Shows Physical State Of Reactants And Products Ppt Download
Chem 101 Acids And Bases Introduction
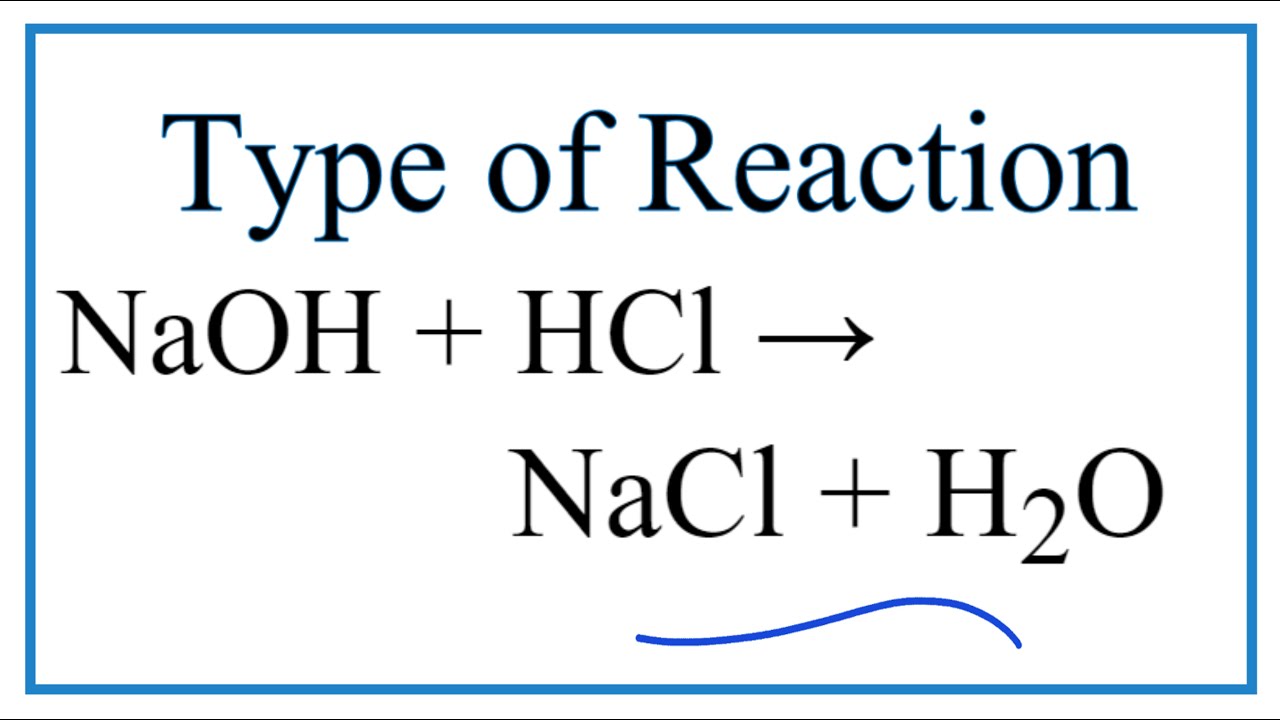
Type Of Reaction For Naoh Hcl Nacl H2o Youtube
A Student Performs A Second Titration In Which The Student Titrates A 20 Ml Sample Of 0 20 M Hcl Aq With 0 10 M Naoh Aq How Many Ml Of 0 10 M Naoh Aq Would Be

How To Balance Naoh Hcl Nacl H2o Sodium Hydroxide Plus Hydrochloric Acid Youtube

Chapter 8 Writing Chemical Equations C 2013 Marshall Cavendish International Singapore Private Limited Ppt Download

Ionic Equations A Chemical Equation Shows The Number Of Atoms And Molecules Of The Reactants And Products Also Shows Physical State Of Reactants And Products Ppt Download

Chemical Equations More Writing And Balancing Practice Go Through This Powerpoint For Extra Tips And Extra Practice Ppt Download

How To Balance Naoh Hcl Nacl H2o Sodium Hydroxide Plus Hydrochloric Acid Youtube
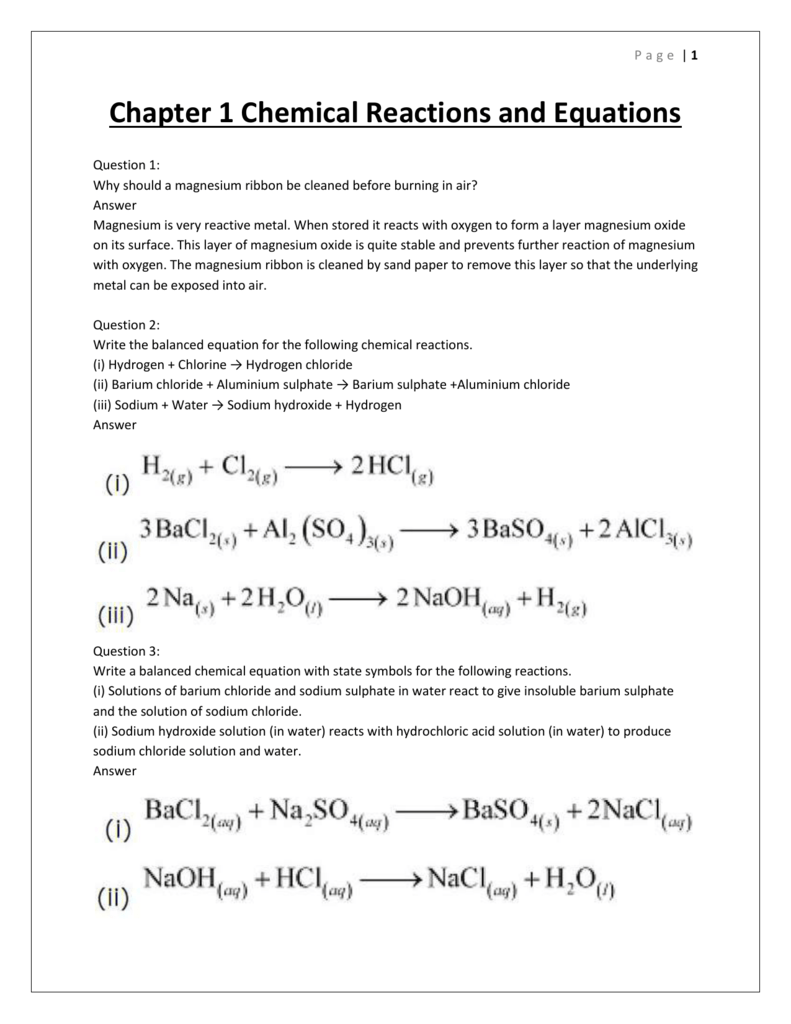
Chemical Reactions And Equations

Naoh Hcl Sodium Hydroxide Hydrochloric Acid Net Ionic Equation Youtube
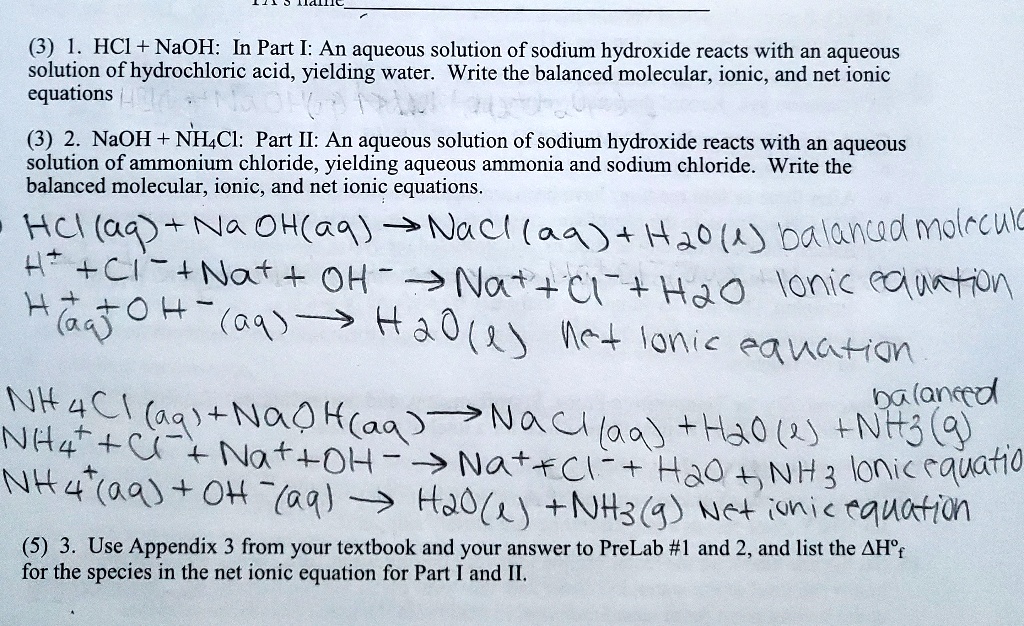
Solved 3 Hci Naoh In Part I An Aqueous Solution Of Sodium Hydroxide Reacts With An Aqueous Solution Of Hydrochloric Acid Yielding Water Write The Balanced Molecular Ionic And Net Ionic Equations

Solved Write The Balanced Chemical Equation For The Reaction Chegg Com
Comments
Post a Comment